CASだ: 10042-76-9
通称: 二硝酸ストロンチウム
分子式: Sr(NO₃)₂
相対分子量: 211.63 g/mol
構造式: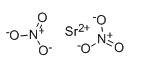
1.製品特性
硝酸ストロンチウムは白色の結晶性粉末で、強い酸化力を持つ。水と液体アンモニアに非常に溶けやすく、エタノールとアセトンにわずかに溶け、硝酸には溶けない。融点は570℃で、高温で分解し、有毒な窒素酸化物を発生する。吸湿性があり、有機物や還元剤と接触すると火災や爆発を起こすことがある。
2.品質基準
| パラメータ | 仕様 |
| 外観 | 白色結晶性粉末 |
| 純度 (Sr(NO₃)₂) | ≥ 99.0% / 99.5% (ar/cp) |
| 水不溶性物質 | ≤ 0.01% (ar), ≤ 0.05% (cp) |
| 乾燥減量 | ≤ 0.1% (ar), ≤ 0.5% (cp) |
| 遊離酸(HNO₃として) | ≤ 0.013% |
| 塩化物(Cl) | ≤ 0.0005% (ar), ≤ 0.002% (cp) |
| 硫酸塩 (SONo_2084) | ≤ 0.005% (ar), ≤ 0.01% (cp) |
| ナトリウム(Na) | ≤ 0.03% (ar), ≤ 0.05% (cp) |
| マグネシウム (Mg) | ≤ 0.005% (ar), ≤ 0.01% (cp) |
| カリウム(K) | ≤ 0.02% (ar), ≤ 0.05% (cp) |
| カルシウム (Ca) | ≤ 0.03% (ar), ≤ 0.05% (cp) |
3.適用範囲と使用方法
硝酸ストロンチウムは、鮮やかな真紅の炎を出すことができるため、赤色信号炎、花火、発炎筒の製造に広く使用されている。硝酸ストロンチウムは、ガラスを精製し、光学特性を向上させるためにガラス産業にも使用されている。電子工学では、正極材料の成分として、また高純度ストロンチウム塩の製造に使われる。さらに、分析化学や先端セラミック材料の合成における前駆体としても利用されている。
4.パッケージと保管
硝酸ストロンチウムは通常、密封されたプラスチックまたはポリエチレンの袋に詰められ、ファイバードラムやプラスチック容器に入れられます。一般的な梱包サイズには、実験室用または工業用の25kg、50kg、500gがある。保管は、熱、湿気、有機化合物、還元剤、酸などの相溶性の悪い物質を避け、涼しく乾燥した換気の良い場所で行う。酸化性で火災の危険性があるため、適切な表示と取り扱いが不可欠である。

-1-300x300.jpg)
.jpg)